Nanophotonics / Nano-Optics (Part II)
Nanophotonics for Molecular Diagnostics and Therapy Applications
Imagen Courtesy: In a typical nanophotonic biosensor, nanostructures on the sensor strongly enhance the incident light field; changes in refractive index at the interface due to biorecognition events (for example, antibody–antigen interactions) are read from the enhanced evanescent field. Maria Soler, Olalla Calvo-Lozano, M.-Carmen Estevez and Laura M. Lechuga (ICN2, Spain)
Nanophotonics deals with the interaction of light with matter at a nanometer scale, providing challenges for fundamental research and opportunities for new technologies, encompassing the study of new optical interactions, materials, fabrication techniques, and architectures, including the exploration of natural and synthetic, or artificially engineered, structures such as photonic crystals, holey fibers, quantum dots, subwavelength structures, and plasmonics . The use of photonic nanotechnologies in medicine is a rapidly emerging and potentially powerful approach for disease protection, detection, and treatment. The high speed of light manipulation and the remote nature of optical methods suggest that light may successfully connect diagnostics, treatment, and even the guidance of the treatment in one theranostic procedure combination of therapeutics with diagnostics (including patient prescreening and therapy monitoring).
Nanophotonics For Diagnostics
Surface Plasmons on Nanoparticles and Surfaces
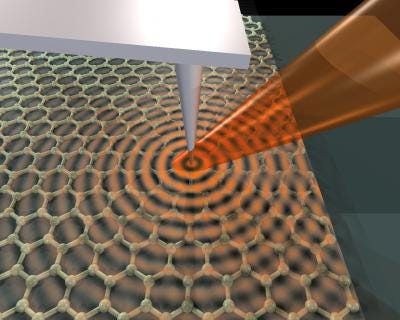
Imagen Courtesy: An infrared laser beam focused on the arm of an atomic-force microscope launches plasmons, waves through electrons, on the surface of graphene, a single honeycomb layer of linked carbon atoms. Dimitri Basov Lab/UCSD
Surface plasmons are collective charge oscillations that occur at the interface between conductors and dielectrics. They can take various forms, ranging from freely propagating electron density waves along metal surfaces to localized electron oscillations on metal nanoparticles (NPs). When light passes through a metal nanoparticle, it induces dipole moments that oscillate at the respective frequency of the incident wave, consequently dispersing secondary radiation in all directions. This collective oscillation of the free conduction electrons is called localized surface plasmon resonance (LSPR). Light on NP induces the conduction electrons to oscillate collectively with a resonant frequency that depends on the nanoparticles’ size, shape, composition, interparticle distance, and environment (dielectric properties). As a result of these SPR modes, the nanoparticles absorb and scatter light so intensely that single NPs are easily observed by eye using dark-field (optical scattering) microscopy. Plasmonic NPs provide a nearly unlimited photon resource for observing molecular binding for longer periods of time, once they do not blink or bleach like fluorophores .
Raman-Spectroscopy-Based Systems
When light interacts with a substance, it can be absorbed, transmitted, or scattered. Scattered radiation can result from an elastic collision (Rayleigh scattering) or inelastic (Raman scattering). Raman spectroscopy is based on a change of frequency when light is inelastically scattered by molecules or atoms resulting in a molecular fingerprint information on molecular structure or intermolecular interaction of a specific process or molecule. The potential of Raman spectroscopy as biomedical diagnostics tool is rather low due to its low cross-section (~10−30 cm2) that results in low sensitivity. However, in 1977, two groups independently described the use of noble metal surfaces to enhance the Raman scattering signal of target molecules —Surface enhancement raman spectroscopy (SERS). Jeanmaire and Van Duyne proposed a twofold electromagnetic field enhancement that was later associated with the interaction between the incident and scattered photons with the nanostructure’s LSPR. Simultaneously, Albrecht and Creighton suggested the source of the enhancement to be caused by a specific interaction between an adsorbate and the nanoparticle surface, briefly, a charge transfer from the adsorbate into the empty energetic levels on the metal surface or from the occupied levels of the nanoparticle’s surface to the adsorbate.
Fluorescence-Based Systems
Quantum dots (QDs) are semiconductor nanoparticles with narrow, tunable, symmetrical emission spectra, and high quantum yields and together with compatibility with DNA and proteins, make QDs exceptional substitutes as fluorescence labels. The use of QDs for nucleic acid characterization has long been proposed, for example, CdSe/ZnS QDs for SNP identification on human TP53 gene, multiallele detection of hepatitis B and C viruses, and in situ detection of chromosome abnormalities and mutations. QDs have also been used as chemical sensors by exploring a typical FRET system where a dark quencher is placed at a protein-binding site attached to a QD surface. The quantum dots emission is quenched in presence of the analyte and upon analyte displacement the emission is restored. A simpler approach was used to detect adenine using fluorescent ZnS nanoparticles at pH7, making use of capability of adenine itself to quench emission of the quantum-dot-like nanoparticles.
Nanophotonics Bioimaging
Imagen Courtesy: INL(Portugal)
Nanoparticles show unique features suitable for biomedical imaging applications, such as an increased sensitivity in detection through amplification of signal changes (e.g., magnetic resonance imaging); high fluorescence quantum yields and large magnetic moments; properties that induce phagocytosis and selective uptake by macrophages (e.g., liposomes); physicochemical manipulations of energy (i.e., quantum dots); among others. Because light absorption from biologic tissue components is minimized at near infrared (NIR) wavelengths, most nanoparticles (e.g., noble metal and magnetic NPs, nanoshells, nanoclusters, nanocages, nanorods and quantum dots) for in vivo imaging and therapy have been designed to strongly absorb in the NIR and used for in vivo diagnostics. Ex vivo and in vivo imaging applications of nanoparticles have included their use as contrast agents for magnetic resonance imaging (MRI), optical coherence tomography (OCT) , photoacoustic imaging (PAI), and two-photon luminescence (TPL) spectroscopy.
Nanophotonics for Therapy
Nanophototerapy uses pulsed lasers and absorbing nanoparticles attached to specific targets for selective damage to cancer cells. Plasmonic photothermal therapy (PPTT) and photodynamic therapy (PDT) are two of the main techniques that take advantage of the selective absorbance of the surface plasmon resonance and the fact that the nanoparticles relax by liberating heat into their surrounding environment.
Plasmonic Photothermal Therapy
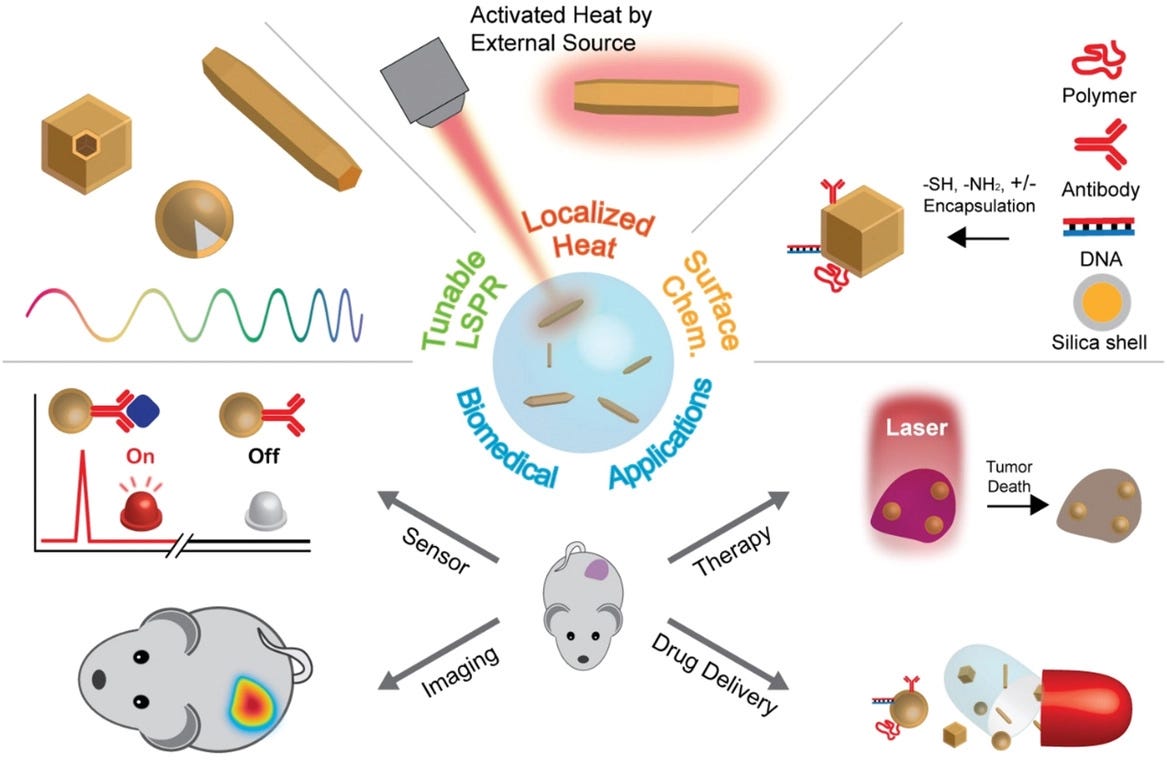
Imagen Courtesy: Representative features of photothermal nanoparticles and their biomedical applications. Jwa-Min Nam. Department of Chemistry, Seoul National University, Seoul, 08826 South Korea
Plasmonic photothermal therapy is a less invasive experimental technique that holds great promise for the treatment of cell malignancies and, in particular, of cancer. It combines two key components: (i) light source, specifically lasers with a spectral range of 650–900 nm for deep tissue penetration and (ii) optical absorption of AuNPs which release the optical irradiation as heat in the picoseconds time scale, thereby inducing photothermal ablation. Kirui et al. reported the use of gold and iron oxide hybrid nanoparticles in targeting, imaging, and selective thermal killing of colorectal cancer cells. Huang and colleagues have demonstrated that gold nanorods have a longitudinal absorption band in the NIR on account of SPR oscillations and are effective as photothermal agents. Gold nanorods aspect ratios allow tuning the SPR band from the visible to the NIR (transmits readily through human skin and tissue), making them suitable for photothermal converters of near infrared light for in vivo applications. Effective photothermal destruction of cancer cells and tissue have been demonstrated for other gold nanostructures, such as branched gold nanoparticles, gold nanoshells, gold nanocages, and gold nanospheres.
Photodynamic Therapy
Photodynamic therapy employs chemical photosensitizers that generate reactive oxygen species (ROS), such as a singlet oxygen (1O2), capable of tumor destruction. This technique is noninvasive and can be applied locally or systemically without noticeable cumulative toxicity effects without high costs. To attain maximal killing efficiency of tumor cells, the photosensitizer must be in close proximity to the tumor cells, thus requiring specific targeting when administered systemically. One of the major limitations is the poor tissue penetration of high-energy light and the systemic dispersal of the photosensitizer.
Don't hesitate to subscribe if you haven't already done so.
Regards and take care.
Born in 2012, Scixel is a project devoted to the improvement of the scientific comunication through the creation of graphical products: pictures, animations, graphs, posters, etc. Scixel consists of scientists with a deep knowledge in digital graphics but also with a long experience in giving talks, preparing posters and papers and other daily situations of scientific work.
We have focused our work into universities and research institutes all over the world: TuDelft (The Netherlands), NIMS (Japan), Basel University (Switzerland), Universidad Autónoma de Madrid, CNB or ICFO (Spain), to name a few.
Web: https://scixel.es/
If you are a company or an individual who would like to place your advertising in my newsletter you can contact me (email) and let me know your request of type of ad and number of newsletters you would like to place it. I will send you a budget as soon as possible.